Breaking News: Guangzhou Corebone Obtains Class III Medical Device Certification
6 June, 2025
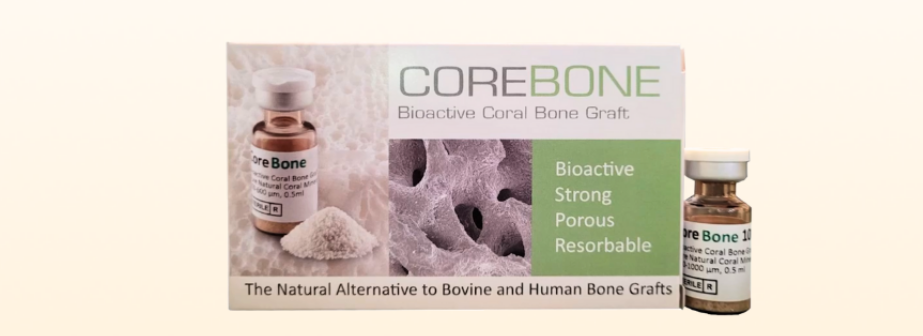
Guangzhou Corebone Medical Technology Co., Ltd. ("the Company"), a portfolio company of GIBF, announced that its dental bone powder product has officially obtained the Class III Medical Device Registration Certificate issued by the National Medical Products Administration (NMPA). This milestone signifies that the company's dental bone powder has met the stringent national standards for safety, efficacy, and quality control, earning the qualification to enter the market and be applied in clinical settings. It brings a new option to the field of domestic oral restoration. Notably, this dental bone powder product employs a novel bone graft technology using farmed coral, marking its first-ever launch in China.
Dental bone powder is widely used in oral medicine, particularly in the field of dental implants. As public awareness of oral health continues to grow and dental implant technology becomes increasingly popular, the demand for high-quality dental bone powder is also rising steadily. The approval of the company’s dental bone powder not only enriches the variety of products available in the domestic market but also provides clinicians with more high-quality treatment options. It is expected to deliver better therapeutic outcomes and an improved quality of life for numerous patients with oral diseases.
Obtaining a Class III Medical Device Registration Certificate is a critical threshold for medical device products to enter the market. The successful approval of the company's dental bone powder demonstrates the company's comprehensive capabilities in product development, production management, and quality control. It also lays a solid foundation for its future growth in the medical device industry.

